Service Highlights
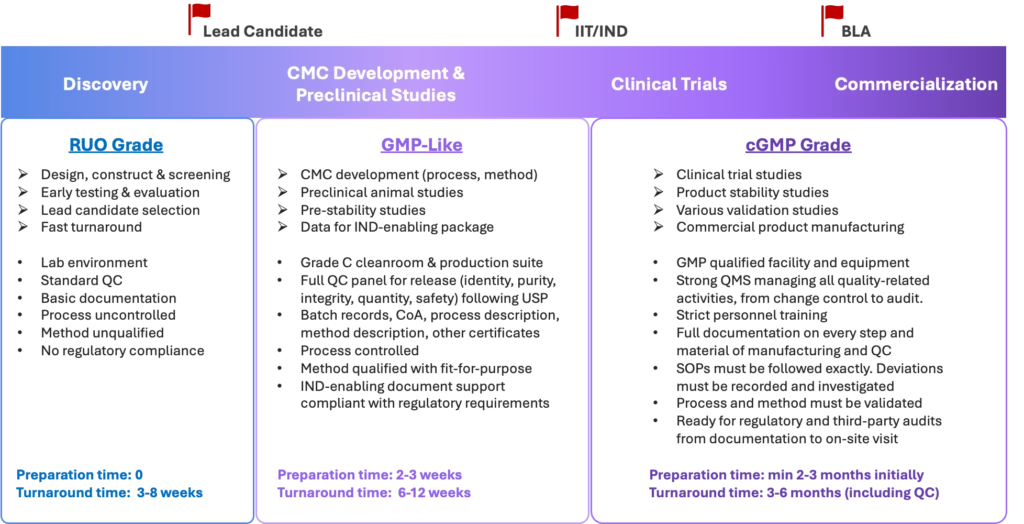
- Standardized manufacturing workflows aligned with GMP principles, without full GMP operational overhead.
- Designed to support advanced preclinical, toxicology, IIT and IND-enabling studies.
- Manufacturing scales adapted to development and IIT/IND needs, enabling rapid material supply.
- Grade C clean room environment with strong emphasis on impurity control, consistency, and batch-to-batch reproducibility.
- Clear process documentation to support regulatory preparation and technology transfer.
-
Built to integrate smoothly with Crystal NAX’s RNA design, LNP formulation, and analytical platforms.
Our Promise
- GMP-Like Process Design
- Early-Phase Manufacturing Readiness
- Flexible Scale & Fast Turnaround
- High-Quality Output
- Process Transparency & Documentation
- Seamless R&D to Development Transition
When to choose GMP-Like?